Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter. It is calculated using the following formula:
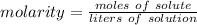
The molarity of the solution is 0.600 M Ba(OH)₂. 1 molar (M) is also equal to 1 mole per liter, so the molarity is 0.600 moles of Ba(OH)₂ per liter.
The volume of the solution is 205 milliliters, however, we need to convert the volume to liters. Remember that 1 liter contains 1000 milliliters.
Now we know the molarity and volume, but the moles are still unknown.
- molarity = 0.600 mol Ba(OH)₂/ L
- moles of solute = x
- liters of solution = 0.205 L
Substitute these values into the formula.
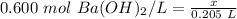
We are solving for x or the moles of solute, so we must isolate the variable. It is being divided by 0.205 liters. The inverse of division is multiplication. Multiply both sides by 0.205 L.
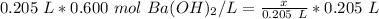
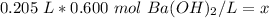
The units of liters cancel.
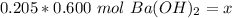
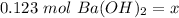
The original measurements had at least 3 significant figures. Our answer currently has 3 sig figs, so we don't need to round.
There are 0.123 moles of barium hydroxide.