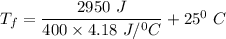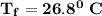Answer:
26.8°C
Step-by-step explanation:
For this reaction, the equation can be computed as follows:
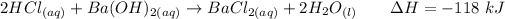
Now, Given that:
100.0 mL of 0.500 molarity (M) of HCl reacted with 300.0 mL of 0.100 molarity of Ba(OH)2.
The next process is to determine the number of moles of the solute:
For HCl:
no of mole =
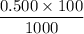
= 0.05 mol
For Ba(OH)2:
no of mole =
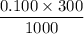
= 0.03 mol
From the reaction, at the reactant side, we will see that two moles of HCl reacted with one mole of Ba(OH)2.
It implies that HCl is the limiting agent since it is in lesser quantity.
Similarly, the heat given out of the system is 118 kJ for each two moles of HCl with one mole of B(OH)2.
Hence, for 0.05 moles of HCl, the heat emitted out is:
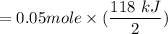
= 2.95 kJ
Let's recall from the question that:
the heat emitted was moved to the final mixture with mass =400g
the specific heat capacity = 4.18 J/°C g
Initial temperature = 25°C
∴
Using the formula;
ΔH = mass × specific heat capacity × (T_f - T_i)
Then, replacing the values, we have:
2.95 kJ = 400 g × 4.18 J/°C g × ( T_f - 25° C)
2950 J = 400 × 4.18 J/°C × ( T_f - 25° C)