Answer:
5.20 grams of Br₂
Step-by-step explanation:
From our previous knowledge;
We understand that:
The number of moles of a given element = mass of the element divided by its molar mass.
Mathematically:
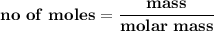
From the given information, let's assume that the 0.065 moles of liquid -bromine partake in the reaction.
From the periodic table, the molar mass of Bromine is = 79.9 g/mol
As such, the mass of liquid that partakes is calculated as:
0.065 mol = mass/ 79.9 g/mol
mass = 0.065 mol × 79.9 g/mol
mass of liquid that partakes in the reaction = 5.20 grams of Br₂