Answer:

Step-by-step explanation:
1. Calculated Final Volume
We are asked to find the final volume of a balloon given a change in temperature. We will use Charles's Law, which states the volume of a gas is directly proportional to the temperature. The formula for this law is:
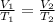
The initial volume is 173.8 milliliters and the initial temperature is 17.5 degrees Celsius.
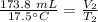
The balloon is heated to a final temperature of 78.0 degrees Celsius, but the volume is unknown.
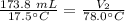
We are solving for the final volume, so we must isolate the variable V₂. It is being divided by 78.0 degrees Celsius. The inverse of division is multiplication, so we multiply both sides by 78.0 °C.
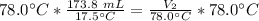
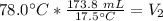
The units of degrees Celsius cancel.
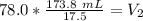
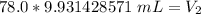
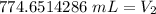
2. Convert to Liters
We are asked to give the volume in liters, so we must convert out units. Remember that 1 liter contains 1000 milliliters.
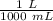
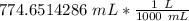
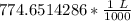
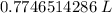
3. Round
The original values of volume and temperature have 3 and 4 significant figures. We always round our answer to the least number of sig figs, which is 3. This is the thousandths place for the number we calculated. The 6 in the ten-thousandths place tells us to round the 4 up to a 5.
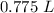
The final volume is approximately 0.775 liters.