Answer: The mass of copper liberated is 0.196 g.
Step-by-step explanation:
The oxidation half-reaction of copper follows:
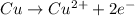
Calculating the theoretical mass deposited by using Faraday's law, which is:
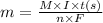 ......(1)
......(1)
where,
m = actual mass deposited = ? g
M = molar mass of metal = 63 g/mol
I = average current = 2 A
t = time period in seconds = 5 min = 300 s (Conversion factor: 1 min = 60 sec)
n = number of electrons exchanged = 2
F = Faraday's constant = 96500 C/mol
Putting values in equation 1, we get:
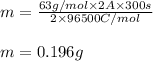
Hence, the mass of copper liberated is 0.196 g.