Step-by-step explanation:
The given data is:
The mass% of a solution is 7.
Mass of solution =450g
The mass of salt required can be calculated as shown below:
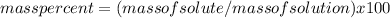
Substitute the given values in this formula to get the mass of solute that is salt:
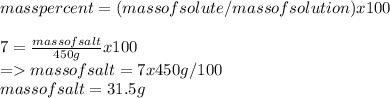
Mass of salt =31.5g
Mass of solvent that is water = 450g-31.5g=418.5g