Answer:
![[O_2]_(eq)=0.030M](https://img.qammunity.org/2022/formulas/chemistry/college/duverg21e0e504lo1hadu2xj8vvurc0d3l.png)
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by firstly writing out the mathematical expression for the concentration of oxygen at equilibrium, given the initial one and the change due to the reaction extent:
![[O_2]_(eq)=0.050M-x](https://img.qammunity.org/2022/formulas/chemistry/college/f071jhp54efsrtayszahhvywz06edp3h7k.png)
Whereas
 can be found considering the equilibrium of SO3:
can be found considering the equilibrium of SO3:
![[SO_3]_(eq)=2x=0.040M](https://img.qammunity.org/2022/formulas/chemistry/college/pkmx5ifmsde2gjko4hanuvdvqkg51wv66p.png)
Which means:
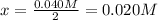
Thus, the equilibrium concentration of oxygen gas turns out:
![[O_2]_(eq)=0.050M-0.020M=0.030M](https://img.qammunity.org/2022/formulas/chemistry/college/15sivze0lbb8girp8uy2y0gfqd5gzon644.png)
Regards!