Answer:
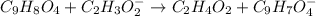
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to figure out the required net ionic equation by firstly writing out the complete molecular equation between aspirin and sodium acetate:
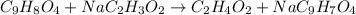
Whereas acetic acid and sodium acetylsalicylate are formed. Now, we write the complete ionic equation whereby sodium acetate and sodium acetylsalicylate are ionized because they are salts yet neither aspirin nor acetic acid are ionized as they are weak acids:
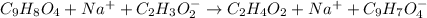
Finally, for the net ionic equation we cancel out the sodium spectator ions to obtain:
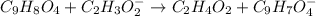
Regards!