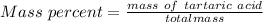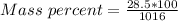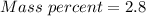Answer:
1)
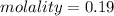
2)
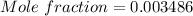
3)
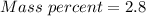
Step-by-step explanation:
Concentration of Tartaric acid=0.190mole /l
1)
Generally
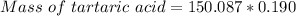
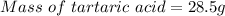
Since 1L of solution tartartic acid is
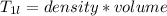
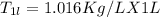
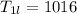
Therefore
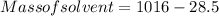
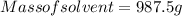
Generally the equation for molality is mathematically given by
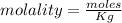
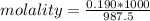
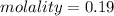
2.
Generally the equation for Moles of water is mathematically given by
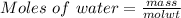
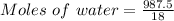
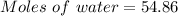
Therefore
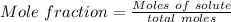
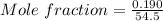
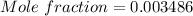
3
Generally the equation for Mass Percent is mathematically given by