Answer:

Step-by-step explanation:
The question asks us to find the new temperature given a change in pressure. We will use Gay-Lussac's Law, which states the pressure of a gas is directly proportional to the temperature. The formula is:
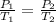
The pressure changes from 767 millimeters of mercury (P₁) to 800 millimeters of mercury (P₂).

The temperature is initially 325 K (T₁), but we don't know the final temperature or T₂.

We are solving for the final temperature, so we must isolate the variable T₂. Cross multiply. Multiply the first numerator by the second denominator, then the first denominator by the second numerator.
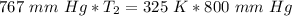
T₂ is being multiplied by 767 millimeters of mercury. The inverse of multiplication is division. Divide both sides by 767 mm Hg.
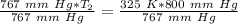
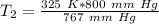
The units of millimeters of mercury (mm Hg) cancel.
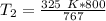
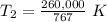
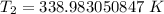
The original measurements have 3 significant figures, so our answer must have the same. For the number we found, that is the ones place. The 9 in the tenths place tells us to round the 8 up to a 9.
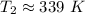
The temperature changes from 325 Kelvin to 339 Kelvin.