Answer:
(a) W = 1329.5 J = 1.33 KJ
(b) ΔU = 24.27 KJ
Step-by-step explanation:
(a)
Work done by the gas can be found by the following formula:
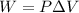
where,
W = Work = ?
P = constant pressure = (0.991 atm)(
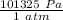 ) = 100413 Pa
) = 100413 Pa
ΔV = Change in Volume = 18.7 L - 5.46 L = (13.24 L)(
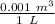 ) = 0.01324 m³
) = 0.01324 m³
Therefore,
W = (100413 Pa)(0.01324 m³)
W = 1329.5 J = 1.33 KJ
(b)
Using the first law of thermodynamics:
ΔU = ΔQ - W (negative W for the work done by the system)
where,
ΔU = change in internal energy of the gas = ?
ΔQ = heat added to the system = 25.6 KJ
Therefore,
ΔU = 25.6 KJ - 1.33 KJ
ΔU = 24.27 KJ