Answer:
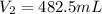
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by using the combined gas law due to the fact that we are dealing with variable volume, temperature and pressure:
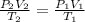
In such a way, we solve for the final volume, V2, considering that the initial volume, V1, is 500 mL, the initial temperature, T1, is 273 K (STP), the initial pressure, P1, is 1 atm (STP) and the final temperature, T2, is 325 K and the final pressure, P2, is 125 kPa (1.23 atm):
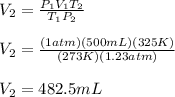
Regards!