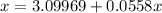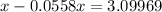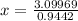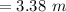Answer:
The correct solution is "3.28 m".
Step-by-step explanation:
According to the question,
Mol fraction of solvent,
= 0.0558
Molar mass of water,
= 18 g/mol
Mol of H₂O in 1000 g water,
= 55.55 mol
Now,
Let the mol of solute will be "x mol".
Total mol in solution will be "55.55 + x".
As we know,
⇒ The mol fraction of solvent =
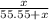
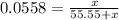
![x=0.0558[55.55+x]](https://img.qammunity.org/2022/formulas/chemistry/college/t967haccf3vebf8j5b9pyzqf0eo7hyqrad.png)