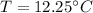Answer:
The correct approach is "12.25°C".
Step-by-step explanation:
Given:
Mass of lead,
mc = 245 g
Initial temperature,
tc = 300°C
Mass of Aluminum,
ma = 150 g
Initial temperature,
ta = 12.0°C
Mass of water,
mw = 820 g
Initial temperature,
tw = 12.0°C
Now,
The heat received in equivalent to heat given by copper.
The quantity of heat =
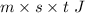
then,
⇒
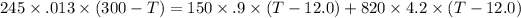
⇒
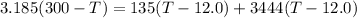
⇒
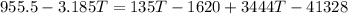
⇒
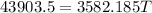
⇒