Answer: If a gas is pumped from a smaller container to a container that is twice the size, and its pressure is kept the same, then temperature of the gas increases twice.
Step-by-step explanation:
Charles law states that at constant pressure, the volume of a gas is directly proportional to the temperature.
That is,
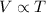 .
.
So, when a gas is pumped from a smaller container to a container that is twice the size shows that volume is increasing by 2 times and its pressure is kept the same.
Therefore,
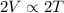
This means that the temperature of the gas will also become twice its initial temperature.
Thus, we can conclude that if a gas is pumped from a smaller container to a container that is twice the size, and its pressure is kept the same, then temperature of the gas increases twice.