Answer: The osmotic pressure of 5.0g of sucrose solution in 1 L is 271.32 torr.
Step-by-step explanation:
Given: Mass = 5.0 g
Volume = 1 L
Molar mass of sucrose = 342.3 g/mol
Moles are the mass of a substance divided by its molar mass. So, moles of sucrose are calculated as follows.
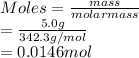
Hence, concentration of sucrose is calculated as follows.
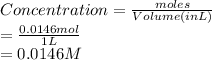
Formula used to calculate osmotic pressure is as follows.

where,
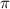 = osmotic pressure
= osmotic pressure
C = concentration
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
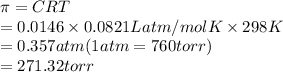
Thus, we can conclude that the osmotic pressure of 5.0g of sucrose solution in 1 L is 271.32 torr.