Step-by-step explanation:
So, first you will want to write the balanced chemical equation for this reaction.
Butane =
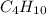
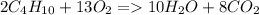
^ This ends up being your balanced chemical equation. Now, you can do the math!
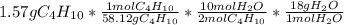
After plugging this into a calculator, your final mass of water should be:
2.43gH2O