Answer:
Ka = 3.45x10⁻⁶
Step-by-step explanation:
First we calculate [H⁺], using the given pH:
- [H⁺] =
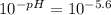
To solve this problem we can use the following formula describing a monoprotic weak acid:
- [H⁺] =

We input the data that we already know:
- 2.51x10⁻⁶ =
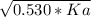
And solve for Ka: