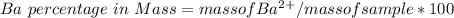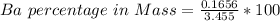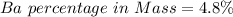Answer:
Step-by-step explanation:
From the question we are told that:
Mass of mixture
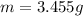
Mass of Barium
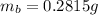
Equation of Reaction is given as
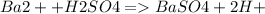
Generally the equation for Moles of Barium is mathematically given by
Since
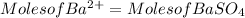
Therefore
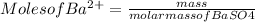
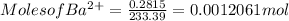
Generally the equation for Mass of Barium is mathematically given by
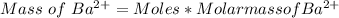
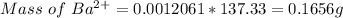
Therefore