Answer: The equation Upper H superscript plus, plus upper O upper H superscript minus right arrow upper H subscript 2 upper O represents the correct net ionic equation for the reaction between
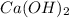 and
and
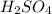 .
.
Step-by-step explanation:
An ionic equation is defined as the equation where both reactants and products are present in the form of ions.
For example, ionic equation between
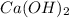 and
and
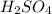 is as follows.
is as follows.
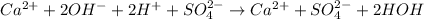
Cancelling the spectator ions then net ionic equation will be as follows.
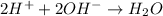
Thus, we can conclude that the equation Upper H superscript plus, plus upper O upper H superscript minus right arrow upper H subscript 2 upper O represents the correct net ionic equation for the reaction between
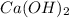 and
and
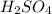 .
.