Answer:
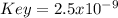
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the equilibrium constant value for the reverse reaction:
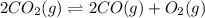
By knowing that the equilibrium expression is actually:
![Key =([CO]^2[O_2])/([CO_2]^2) =(1)/(Keg)](https://img.qammunity.org/2022/formulas/chemistry/college/9asts6onn7ij0mctgbfymd80ms8qogkwkn.png)
Thus, we plug in and solve for the inverse of Keq to obtain Key as follows:
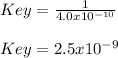
Regards!