Answer:
A. 31.0
Step-by-step explanation:
Remember the formula
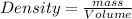 , or
, or
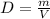 .
.
The problem tells us that the density is 1.89 g/L, and that the volume is 16.4 L.(Remember that if the units are in some form of liters, it is volume, similar to how if the units are in grams, it is mass.)
Plug in the numbers for density and volume, and you will get:
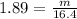
Then, multiply both sides by 16.4 to get:
m = 30.996 g ≈ 31.0 g
Hope this helps!