Answer: Balanced equation that is formed by combining these two half reactions is
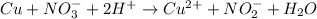 .
.
Step-by-step explanation:
A chemical equation which contains same number of atoms on both reactant and product side is called a balanced chemical equation.
For example,
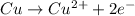
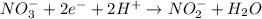
On cancelling the common species from both these half-reactions, the complete balanced equation will be as follows.
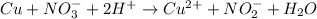
Thus, we can conclude that balanced equation that is formed by combining these two half reactions is
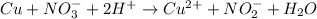 .
.