Answer:
The range of the photon energies is between:
2.652 x 10⁻²⁵ J to 4.973 x 10⁻²⁵ J
Step-by-step explanation:
The energy of a photon is calculated using the following equation;
E = hf
where;
h is Planck's constant = 6.63 x 10⁻³⁴ Js
f is frequency of the photon
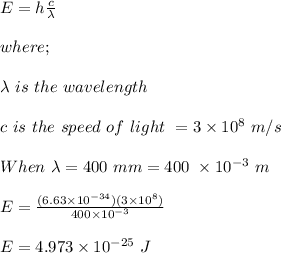
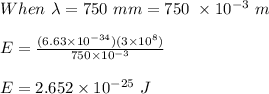
The range of the photon energies is between:
2.652 x 10⁻²⁵ J to 4.973 x 10⁻²⁵ J