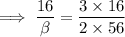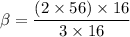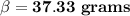Answer:
37.33 grams
Step-by-step explanation:
The missing information embedded in the idea section is attached in the image below:
The aim of this question is to determine the atomic wt. of Iron (Fe) from the hypothetic formula:
Fe₁O₁
Here, we know that the mole ratio can be written as:
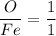
Suppose we assume that the atomic wt. of Fe = β(unknown)???
Then the grams of O and Fe that is contained in Fe₁O₁ can be expressed as:
For O:
1 × 16 grams of Oxygen = 16 grams of O
For Fe:
1 × β grams of Fe = β grams of Fe
Now, let's take a look at the idea experiment, the mole solution can be computed as:
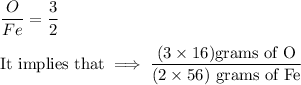
Equating both expressions above, we have: