Answer: The pH of the resulting solution will be 3.001
Step-by-step explanation:
Molarity is calculated by using the equation:
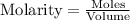 ......(1)
......(1)
We are given:
Moles of NaOH = 0.0224 moles
Molarity of nitrous acid = 0.475 M
Molarity of sodium nitrite = 0.302 M
Volume of solution = 150 mL = 0.150 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
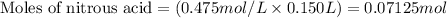
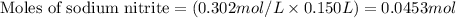
The chemical equation for the reaction of nitrous acid and NaOH follows:
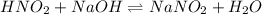
I: 0.07125 0.0224 0.0453
C: -0.0224 -0.0224 +0.0224
E: 0.04885 - 0.0677
The power of the acid dissociation constant is the negative logarithm of the acid dissociation constant. The equation used is:
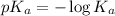 ......(2)
......(2)
We know:
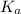 for nitrous acid =
for nitrous acid =
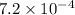
Using equation 2:
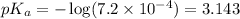
To calculate the pH of the acidic buffer, the equation for Henderson-Hasselbalch is used:
![pH=pK_a+ \log \frac{\text{[conjugate base]}}{\text{[acid]}}](https://img.qammunity.org/2022/formulas/chemistry/college/mmqbcqjf8qsyvpilhkgby8x0ebxkeuuvve.png) .......(3)
.......(3)
Given values:
![[NaNO_2]=(0.0677)/(0.150)](https://img.qammunity.org/2022/formulas/chemistry/college/nebb7rque3lr3tqufgq9t6dymokm0i28xr.png)
![[HNO_2]=(0.04885)/(0.150)](https://img.qammunity.org/2022/formulas/chemistry/college/6xxf8sezpqmdj2u3d3lj4l69jg7gsyuxqj.png)
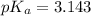
Putting values in equation 3. we get:
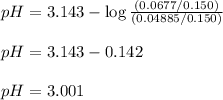
Hence, the pH of the resulting solution will be 3.001