Answer:
a) The volume of oxygen used for combustion at STP is approximately 305 dm³
b) The volume of gas released during combustion at STP is approximately 508 dm³
Step-by-step explanation:
The given chemical reaction equation for the burning of ethanol in air, is presented as follows;
2CH₃CH₂OH (l) + 6O₂ (g) → 4CO₂ (g) + 6H₂O
The mass of ethanol used up in the combustion process, m = 209 g
The molar mass of ethanol, MM = 46.06844 g/mol∴
The number of moles of ethanol in the reaction, n = m/MM
∴ n = 209 g/(46.06844 g/mol) ≈ 4.537 moles
a) Given that 2 moles of ethanol, CH₃CH₂OH reacts with 6 moles of oxygen gas molecules, O₂, 4.54 moles of ethanol will react with (6/2) × 4.537 = 13.611 moles of oxygen
The volume occupied by one mole os gas at STP = 22.4 dm³
The volume occupied by the 13.611 moles of oxygen gas at STP, 'V', is given as follows;
V = 13.611 mol × 22.4 dm³/mole = 304.8864 dm³ ≈ 305 dm³
The volume occupied by the 13.611 moles of oxygen gas at STP, V = The volume of oxygen used for the combustion ≈ 305 dm³
b) The total number of moles of gases released in the reaction, is given as follows;
The total number of moles = (4.537/2) × (4 + 6) = 22.685 moles of gas
The total volume of gas released,
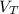 = The volume of gas released during the combustion at STP = 22.685 moles × 22.4 dm³/mole = 508.144 dm³ ≈ 508 dm³
= The volume of gas released during the combustion at STP = 22.685 moles × 22.4 dm³/mole = 508.144 dm³ ≈ 508 dm³