Answer:
The value for the equilibrium constant for the reaction
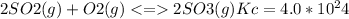 at 298K .
at 298K .
What would be the value for the equilibrium constant for the following reaction at the same temperature?
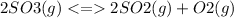
Step-by-step explanation:
The Kc value for the reverse reaction of the first reaction that is:
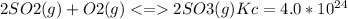
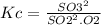
For the reverse reaction,
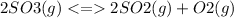
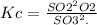
So, it is the inverse of the first Kc value.
Hence, the new Kc value is:
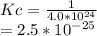
Answer is :
Kc=2.5*10^-25.