Answer:
A volumetric pipette is used to measure 25.0cm3 of 2.0mol/dm3 aqueous sodium hydroxide into aconical flask.
A burette is filled with dilute sulfuric acid.
Write a balanced symbol equation for the reaction between these chemicals when we neutralise.
Step-by-step explanation:
The reactants of the given reaction are:
sodium hydroxide and dilute sulfuric acid.
The balanced chemical equation of the reaction is:
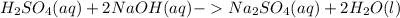
Acid and base raect with each other and form salt and water.
This reaction is called a neutralization reaction.