Answer:
O2 is the limiting reactant.
Step-by-step explanation:
Hello there!
In this case, according to the given information about this reaction, one could identify the limiting reactant by performing a mole ratio of H2S to SO2 and O2 to SO2:
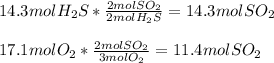
Thus, since 17.1 moles of O2 yields fewer moles than 14.3 moles of H2S, we infer the former is the limiting reactant.
Regards!