Answer:
The given molecules are SO2 and BrF5.
Step-by-step explanation:
Consider the molecule SO2:
The central atom is S.
The number of domains on S in this molecule is three.
Domain geometry is trigonal planar.
But there is a lone pair on the central atom.
So, according to VSEPR theory,
the molecular geometry becomes bent or V-shape.
Hybridization on the central atom is
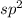 .
.
Consider the molecule BrF5:
The central atom is Br.
The number of domains on the central atom is six.
Domain geometry is octahedral.
But the central atom has a lone pair of electrons.
So, the molecular geometry becomes square pyramidal.
The hybridization of the central atom is
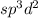 .
.
The shapes of SO2 and BrF5 are shown below: