Answer:
a) 0.15 mol.
b) 8.95 g.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to infer this problem is solved by using the ideal gas equation:

And proceed as follows:
a) Here, we solve for the moles, n, as follows:

b) for the calculation of the mass, we recall the molar mass of butane, 58.12 g/mol, to obtain:
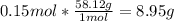
Regards!