Answer:
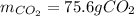
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out mandatory for us to calculate the reacting moles of both C and O2 because we are given grams and pressure, temperature and volume, respectively:
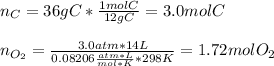
Thus, since C and O2 react in a 1:1 mole ratio, we infer C is in excess, and the grams of CO2 can be calculated with the moles of O2:
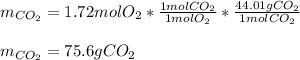
Best regards!