Answer:
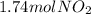
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to perform mole-mass relationships by using the molar mass of the involved substance, in this case NO2, which is 46.0 g/mol; then we just set up a conversion factor like the one shown below:
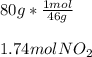
Regards!