Answer:

Step-by-step explanation:
The temperature and volume of the gas are changing, so we use Charles's Law. This states the temperature of a gas is directly proportional to the volume of a gas. The formula is:
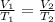
The original volume is unknown. The new volume is 75 milliliters.
The gas is cooled from 150 Kelvin to 50 Kelvin, so the original temperature is 150 K and the new temperature is 50 K.
We know that:
- T₁= 150 K
- V₂= 75 mL
- T₂= 50 K
Substitute the values into the formula.
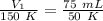
Since we are solving for the original volume, we must isolate the variable V₁.
It is being divided by 150 K. The inverse of division is multiplication, so we multiply both sides by 150 K.

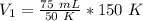
The units of Kelvin (K) cancel.
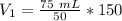
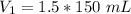
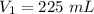
The original volume is 225 milliliters.