Answer: There are
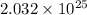 molecules
molecules
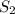 gas are in 756.2 L.
gas are in 756.2 L.
Step-by-step explanation:
It is known that 1 mole of any gas equals 22.4 L at STP. Hence, number of moles present in 756.2 L are calculated as follows.
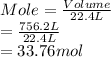
According to mole concept, 1 mole of every substance contains
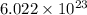 molecules.
molecules.
Therefore, molecules of S present in 33.76 moles are calculated as follows.
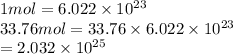
Thus, we can conclude that there are
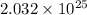 molecules
molecules
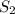 gas are in 756.2 L.
gas are in 756.2 L.