Answer:
a) the most probable speed of the molecules is 409.2 m/s
b) required temperature of xenon is 1322 K
Step-by-step explanation:
Given the data in the question;
a)
Maximum probable speed of hydrogen molecule (H₂)
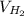 = √( 2RT /
= √( 2RT /
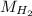 )
)
where R = 8.314 m³.Pa.K⁻¹.mol⁻¹ and given that T = 20.3 K
molar mass of H₂;
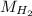 = 2.01588 g/mol
= 2.01588 g/mol
we substitute
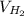 = √( (2 × 8.314 × 20.3 ) / 2.01588 × 10⁻³ )
= √( (2 × 8.314 × 20.3 ) / 2.01588 × 10⁻³ )
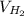 = √( 337.5484 / 2.01588 × 10⁻³ )
= √( 337.5484 / 2.01588 × 10⁻³ )
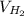 = 409.2 m/s
= 409.2 m/s
Therefore, the most probable speed of the molecules is 409.2 m/s
b)
Temperature of xenon = ?
Temperature of hydrogen = 20.3 K
we know that;
T = (Vxe² × Mxe) / 2R
molar mass of xenon; Mxe = 131.292 g/mol
so we substitute
T = ( (409.2)² × 131.292 × 10⁻³) / 2( 8.314 )
T = 21984.14167 / 16.628
T = 1322 K
Therefore, required temperature of xenon is 1322 K