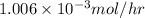Answer:
For A: The average molecular speed of Ne gas is 553 m/s at the same temperature.
For B: The rate of effusion of
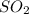 gas is
gas is
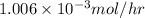
Step-by-step explanation:
For A:
The average molecular speed of the gas is calculated by using the formula:
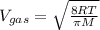
OR
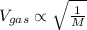
where, M is the molar mass of gas
Forming an equation for the two gases:
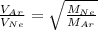 .....(1)
.....(1)
Given values:
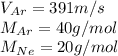
Plugging values in equation 1:
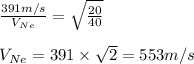
Hence, the average molecular speed of Ne gas is 553 m/s at the same temperature.
For B:
Graham's law states that the rate of diffusion of a gas is inversely proportional to the square root of the molar mass of the gas. The equation for this follows:
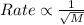
Where, M is the molar mass of the gas
Forming an equation for the two gases:
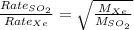 .....(2)
.....(2)
Given values:
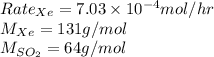
Plugging values in equation 2:
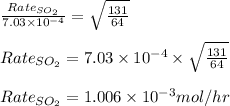
Hence, the rate of effusion of
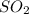 gas is
gas is