Answer:
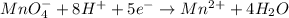
Step-by-step explanation:
Hello there!
In this case, since redox reactions are characterized by the presence of a reduction reaction, whereby the oxidation of the element decreases, and an oxidation reaction whereby the oxidation of the element increases.
In such a way, for the given chemical equation, we can see Fe is increasing its oxidation state from 2+ to 3+, which means it is oxidized. On the flip side, Mn is being reduced from 7+ (MnO₄⁻) to 2+ and this, the reduction half-reaction is:
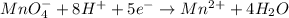
Whereas five electrons are carried.
Regards!