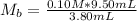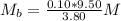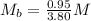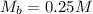Answer:
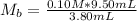 ---- The setup
---- The setup
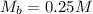 --- The molarity of KOH
--- The molarity of KOH
Step-by-step explanation:
Given
I will answer the question with the attached titration data
Required
The set and the value of the molarity of KOH
First, calculate the volume of acid (HCL) used:
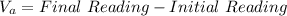
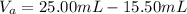
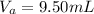
Calculate the final volume of base (KOH) used:
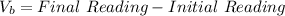
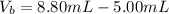
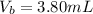
The numerical setup is calculated using::
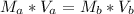
Where
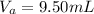
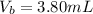
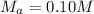 --- the given molarity of HCL
--- the given molarity of HCL
So, we have:
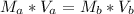
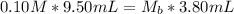
Make Mb the subject
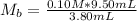 ---- The correct numerical setup
---- The correct numerical setup
The solution is then calculated as: