Answer: The value of
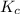 for the given chemical equation is 0.0457.
for the given chemical equation is 0.0457.
Step-by-step explanation:
Given values:
Initial moles of
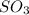 = 0.700 moles
= 0.700 moles
Volume of conatiner = 3.50 L
The given chemical equation follows:
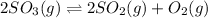
I: 0.700
C: -2x +2x x
E: 0.700-2x 2x x
Equilibrium moles of
 = x = 0.180 moles
= x = 0.180 moles
Equilibrium moles of
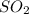 = 2x =
= 2x =
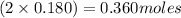
Equilibrium moles of
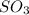 = 0.700 - 2x =
= 0.700 - 2x =
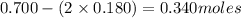
Molarity is calculated by using the equation:
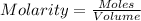
So,
![[SO_3]_(eq)=(0.340)/(3.50)=0.0971M](https://img.qammunity.org/2022/formulas/chemistry/college/wdpq0g9h5vg3nub6iisecuazuta5x6rvz4.png)
![[SO_2]_(eq)=(0.360)/(3.50)=0.103M](https://img.qammunity.org/2022/formulas/chemistry/college/l9f3zy2gzvo5hf0fsvrxd9el93d0bisttb.png)
![[O_2]_(eq)=(0.180)/(3.50)=0.0514M](https://img.qammunity.org/2022/formulas/chemistry/college/dozrnhch0a1zjag4xujlk0py75hz140gms.png)
The expression of
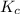 for above equation follows:
for above equation follows:
![K_c=([SO_2]^2[O_2])/([SO_3]^2)](https://img.qammunity.org/2022/formulas/chemistry/college/8c05249s4n6wg6laxrue0qwna7zrj6e5t5.png)
Plugging values in above expression:
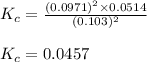
Hence, the value of
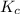 for the given chemical equation is 0.0457.
for the given chemical equation is 0.0457.