English Translation :
A gas at a temperature of 38 °C, have a volume of 21 Lts Liters. What volume will it have if the temperature rises to 67°C?
Solution :
Since, there is no information about pressure, let us assume it is constant.
So, by ideal gas equation at constant pressure :
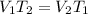
Putting given volume and temperature ( in Kelvin ) in above equation, we get :
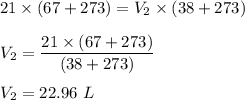
Hence, this is the required solution.