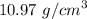Answer:
Step-by-step explanation:
Given :
Mass of a bar of lead = 115.2 g
Initial water level
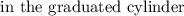 = 25 mL
= 25 mL
Final water level
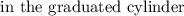 = 35.5 mL
= 35.5 mL
Difference in the water level = 35.5 - 25
= 10.5 mL
=
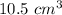
We know that when a body is submerged in water, it displaces its own volume of water.
Therefore, the volume of the lead bar = volume of the water displaced = 10.5 mL =
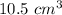
We know that mathematically, density is the ratio of mass of body to its volume.
Density of the lead bar is given by :
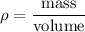
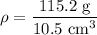
=