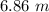Answer:
The molality is "6.86 m".
Step-by-step explanation:
Given:
Mass of of HCL,
= 25 g
Mass of water,
= 100 g
Density,
= 1.19 g/mL
Total mass of solution,
= 125 g
Now,
The number of moles of HCl will be:
=
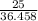
=
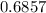
The solution volume will be:
=
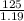
=
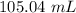
hence,
The molality will be:
=
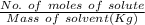
=
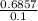
=
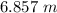
or,
=