Answer:
V = 25.7 L.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve for the volume of hydrogen, by firstly calculating the moles of this gas produced by 37.6 g of magnesium, according to the 1:1 mole ratio between them:
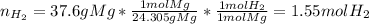
Now, we use the ideal gas equation:

To solve for the volume, and obtain:
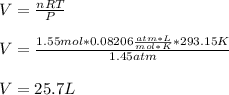
Regards!