Answer:
0.616 moles of Fe₂(CO₃)₃ are produced when 200 g of FeCl₃ react
b. 1.
Step-by-step explanation:
The balanced reaction is:
- 2FeCl₃ + 3(NH₄)₂CO₃ → 6NH₄Cl + Fe₂(CO₃)₃
First we convert 200 grams of FeCl₃ into moles, using its molar mass:
- 200 g ÷ 162.2 g/mol = 1.23 mol FeCl₃
Then we convert 1.23 moles of FeCl₃ into moles of Fe₂(CO₃)₃, using the stoichiometric coefficients of the balanced reaction:
- 1.23 mol FeCl₃ *
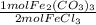 = 0.616 mol Fe₂(CO₃)₃
= 0.616 mol Fe₂(CO₃)₃
The closest answer would be option b. 1.