Answer:
Molality of solution=10.11 m
Step-by-step explanation:
We are given that
Given mass of KCl(WB)=75.3 g
Given mass of water (WA)=100 g=100/1000=0.1 kg
1 kg=1000 g
Molar mass of H=1.01 g
Molar mass of K=39g
Molar mass of Cl=35.45 g
We have to find the molality of a solution.
Molar mass of KCl(MB)=39+35.45
Molar mass of KCl(MB) =74.45 g
Number of moles of solute (KCl)=
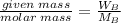
Number of moles of solute (KCl)=
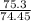
Number of moles of solute (KCl)=1.011 moles
Molality of solution
=
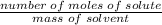
Using the formula
Molality of solution=
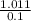
Molality of solution=10.11 m