Answer: The volume of the balloon up there is 6.192 L.
Step-by-step explanation:
Given:
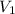 = 1.80 L,
= 1.80 L,
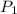 = 785 mm Hg (mm Hg = 0.00131579) = 1.032 atm
= 785 mm Hg (mm Hg = 0.00131579) = 1.032 atm
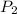 = 0.300 atm,
= 0.300 atm,
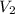 = ?
= ?
Formula used to calculate volume is as follows.
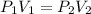
Substitute the value into above formula as follows.
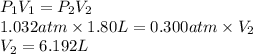
Thus, we can conclude that the volume of the balloon up there is 6.192 L.