Answer:

Step-by-step explanation:
If we want to convert from grams to moles, the molar mass is used. This is the mass of 1 mole. They are found on the Periodic Table as the atomic masses, but the units are grams per mole (g/mol) instead of atomic mass units (amu).
Look up the molar mass of carbon.
Set up a ratio using the molar mass.
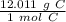
Since we are converting 3.06 grams to moles, we multiply by that value.
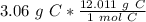
Flip the ratio. This way, the ratio is still equivalent, but the units of grams of carbon cancel.
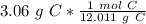
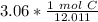
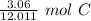
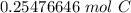
The original measurement of grams (3.06) has 3 significant figures, so our answer must have the same. For the number we calculated, that is the thousandth place.
The 7 in the ten-thousandth place tells us to round the 4 up to a 5.
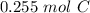
3.06 grams of carbon is approximately 0.255 moles of carbon.