Answer:
The minimum mass of ethylene glycol = 6.641 Kg
Step-by-step explanation:
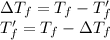
Where T_f = freezing point of pure solvent water, 0°C
T_f'= Freezing point of solvent after mixture
K_f = Freezing point depression constant = 1.86 °C/m
Moecular weight of ethylene glycol = 60 g/mol
Weight of ethylene glycol = 14.5 Kg= 14.5×10^3 g
molality of ethylene glycol
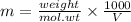
Substitute the values to calculate m
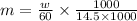
by formula
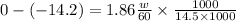
calculating we get w = 6641.93 g
Therefore, The minimum mass of ethylene glycol = 6.641 Kg